Coarctation of the Aorta
Josh Kailin, MD
Coarctation of the aorta (CoA) is a narrowing of the aortic arch. It most commonly occurs in the region of the aortic isthmus (between the left subclavian artery and ductus arteriosus). Patients with a severe coarctation of the aorta are dependent on a patent ductus arteriosus (PDA) to preserve systemic blood flow and perfusion. CoA can be present with an isolated narrowing of the aortic isthmus or with tubular hypoplasia of the aortic arch (transverse arch and isthmus). CoA has a variable spectrum of severity, ranging from unequivocal ductal-dependent systemic blood flow, to patients with borderline small arch dimensions who also have a PDA. In the latter, the determination of true CoA is a challenge even after birth. The sensitivity of fetal diagnosis of CoA varies from 50% to 72%, and delayed diagnosis of CoA is still common. Infants who elude prenatal or early neonatal diagnosis may present in cardiogenic shock that may be associated with profound ventricular dysfunction.
4%–6% of all CHD in the United States
Prevalence of ~4 per 10 000 live births
Genetic Considerations
Turner Syndrome
Associated Lesions and Extracardiac Findings
- Left SVC to coronary sinus
- TAPVR
- Shone’s Complex or Shone’s Variants (defined as fetal z-score <-2)
- Bicuspid/hypoplastic aortic valve
- Narrowed or tunnel-like LVOT, subaortic membrane, VSD with posterior malalignment of outlet septum
- Mitral valve dysplasia/hypoplasia with stenosis
- Left ventricular hypoplasia
- Ventricular septal defect (arch hypoplasia even more common with VSD with posterior malalignment of outlet septum
- Congenital diaphragmatic hernia (CDH)
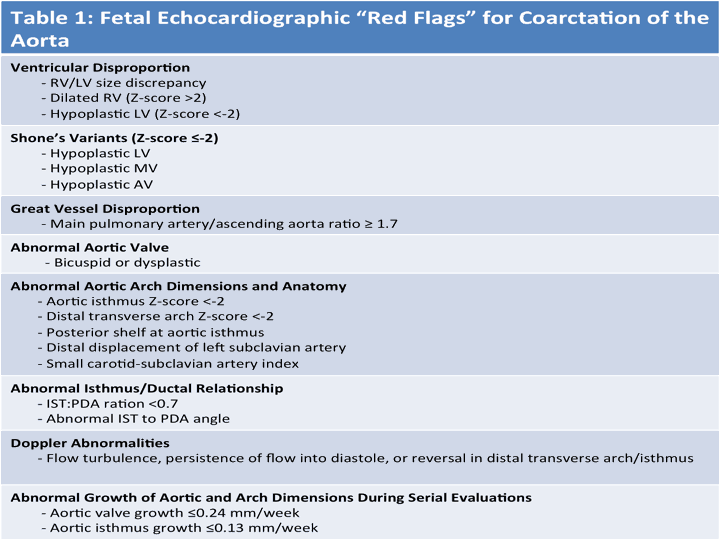
Fetal Imaging Pearls
- Assess for ventricular disproportion from the apical 4 chamber and short axis views. RV predominance is common in the third trimester, but generally should not exceed an RV:LV ratio >1.5
- Evaluate for size discrepancy in the size of the great vessels (noted on three vessel-trachea view) as a marked size difference between the ascending aorta (AAo) and pulmonary artery (PA) can be associated with CoA. A ratio between the PA and AAo of ≥1.7 was found to be associated with CoA, especially in fetuses >28 weeks’ gestation. Size differences likely are a reflection of a redistribution of blood flow secondary to increased resistance in the left ventricular outflow tract with reduced blood flow through the AAo and a compensatory increase in blood flow through the right outflow tract across the PA and PDA in cases of CoA
- Assess mitral valve size, architecture by 2D, color and spectral Doppler
- Assess aortic valve size/morphology with interrogation by color and spectral Doppler
- Detailed quantification of aortic dimensions obtained at the level of the aortic valve annulus, ascending aorta (AAo), transverse aortic arch, isthmus, and descending aorta. The measurements are taken from leading edge to leading edge (internal vessel wall) at their maximal dimension obtained in systole, when the aortic valve is open.
- Color and spectral Doppler examination is performed at the level of the aortic valve, transverse aortic arch, isthmus, descending aorta, and ductal arch. The aortic valve should demonstrate antegrade flow and the velocity time integral and peak velocity recorded. Antegrade flow by color Doppler throughout the entire aortic arch, isthmus, and descending aorta as well as right to left shunting across the PDA should be demonstrated. A step- up in velocity, continuous flow, or flow reversal across the aortic isthmus all warrant more detailed evaluation for the possibility of CoA.
- Hypoplasia of the distal transverse arch and aortic isthmus with z scores <−2, the presence of a posterior shelf in the region of the aortic isthmus and distal displacement of the left subclavian artery are characteristic echocardiographic features in fetal CoA
- A recent meta-analysis revealed that aortic arch hypoplasia had the highest sensitivity (90%) in the diagnosis of coarctation, while a posterior shelf had the highest specificity
- The carotid-subclavian artery index, which is the ratio of the aortic arch diameter at the level of the left subclavian artery, to the distance between the left carotid artery and the left subclavian artery, is smaller in patients with coarctation
Fetal Surveillance
- Depending on the gestational age at the time of initial suspicion, fetal echocardiograms may be performed every 4–6 weeks, with the last evaluation obtained after 30 weeks. At the very least, one follow-up evaluation in the third trimester is reasonable to assess for progression of abnormalities and to detect those that may not have been evident on the initial scan. An important echocardiographic predictor for CoA is progression in the degree of hypoplasia of the LV and aortic dimensions during serial evaluations
- Surveillance should include assessment of left heart growth (LV, mitral valve, aortic valve, aortic arch) with careful attention paid to detailed quantification of aortic dimensions and Doppler assessment of the left heart and aortic arch. This will assist the clinician to more reasonably predict postnatal expectations and whether or not PGE would be required postnatally
Prostaglandins (PGE)
- Yes.
- For the fetus deemed to have ductal- dependent systemic blood flow, delivery at an institution capable of administering prostaglandin (PGE) and address its complications is important. If not possible, arrangements for neonatal transport must be determined prior to delivery.PGE should be initiated following delivery until a postnatal echocardiogram can be performed to assess left heart anatomy and aortic arch
- If the diagnosis is unclear, the neonate may be observed without PGE, and the PDA allowed to close. Active surveillance should be performed and include assessment of right upper and lower extremity pulses for brachialfemoral delay and decreased femoral pulses, a blood pressure gradient between the right arm and the legs, and/or narrowed aortic arch by interval echocardiograms until the diagnosis of CoA is certain (“arch watch”). This surveillance is best performed in the hospital to avoid the development of critical coarctation and cardiogenic shock at home, potentially putting the patient at risk of significant morbidity and even death. Patients without CoA after PDA closure are discharged but reassessed over the first year as arch obstruction may progress with continued tissue constriction in the periductal area.