Truncus Arteriosus
Betul Yilmaz Furtun, MD
Truncus arteriosus (TA) is a rare form of congenital heart disease occurring in 1-3% of patients with congenital heart disease. Truncus arteriosus has an estimated birth incidence of approximately 7 to 21 per 100,000 live births.
During fetal development, the embryonic truncus arteriosus gives rise to the aorta and the pulmonary trunk. Persistent truncus arteriosus results from incomplete or failed septation. It is characterized by a single great artery arising from the heart with a single semilunar valve that overrides the right and left ventricles. The common trunk gives rise to the pulmonary arteries, providing systemic, pulmonary, and coronary perfusion. It may be confused with PA-VSD or aortic atresia with VSD on fetal cardiac evaluation, because one of the great arteries in those conditions is severely hypoplastic.
A ventricular septal defect (VSD) is most commonly associated with TA, as well as anomalies with truncal valve, aortic arch and coronary arteries (outlined below). A VSD is present in the vast majority of cases. It is usually large and conoventricular. Truncal valve leaflets are usually thickened. The valve is most commonly tricuspid, but may also be bicuspid, quadricuspid or pentacuspid (rare) valve.
Approximately 30% of patients with TA have a right aortic arch and 10-12% have aortic arch hypoplasia or an interrupted aortic arch. Coronary artery anomalies seen in TA cases include atypical origin, single coronary, or narrowed ostia resulting in coronary stenosis.
Anatomy/Classification Systems:
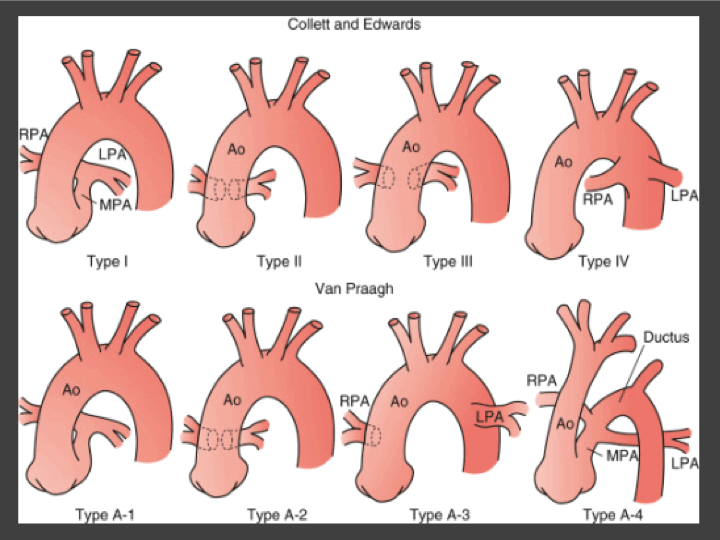
Figure 1: Classifications of truncus arteriosus. Ao, ascending aorta; LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery. (Adapted from St Louis, JD: Persistent truncus arteriosus. In Nichols DG, Ungerleider RM, Spevak PJ, et al [eds]: Critical heart disease in infants and children, Philadelphia, 2006, Mosby, p 690.)
There are four subtypes of truncus arteriosus which are described using two different classification systems (outlined below). Type I/A1 is the most common form, found in ~60% of patients with Truncus Arteriosus.
The Collett and Edwards system is the earliest form of classification, developed in 1949. It is based on where pulmonary arteries arise from the common trunk.
•Type I: The main pulmonary is present and bifurcates into the left and right pulmonary arteries (most common) (same as Van Praagh type A1)
•Type II: the right and left branches arise adjacent to each other from the posterolateral segment of the common trunk (second most common) (same as Van Praagh type A2)
•Type III: The right and left branches originate separately from the right and left lateral segments of the common trunk (as opposed to posterior trunk) (this type is included in Van Praagh type A2).
•Type IV: Neither of the branches arise from the common trunk, but are perfused by aortopulmonary collaterals. This type is now categorized as a form of pulmonary atresia with a ventricular septal defect rather than TA.
The Van Praagh classification system is based on where the branch pulmonary arteries arise from the trunk as well as the development of the aortic arch and the presence of a patent ductus arteriosus (PDA). Each type may include a modifier “A” (with VSD) or “B” (intact ventricular septum).
•Type A1: The main pulmonary is present and bifurcates into the left and right pulmonary arteries (same as Collette and Edwards classification) (most common).
•Type A2: The right and left branch pulmonary arteries arise from the common trunk (second most common). Of note, types A1 and A2 may be difficult to distinguish.
•Type A3: One branch pulmonary artery (typically the right) arises from the common trunk and the other arises from a PDA or the aorta (rare).
•Type A4: This type is defined by presence of aortic arch hypoplasia, coarctation or interrupted aortic arch and a large PDA.
Genetic Considerations:
Approximately 50% of newborns with truncus arteriosus have associated genetic disorder, most commonly the chromosome 22q11.2 deletion syndrome (~20% of the cases). Truncus arteriosus has also been described in trisomy 18, trisomy 21, 14q deletion, GATA6 mutations, and chromosome 3q22.3 deletions.
Fetal Imaging Pearls:
- The four-chamber can be misleading as it appears normal. TA can be detected on the outflow tract views when only one arterial trunk is identified overriding a large VSD.
- Identifying one arterial trunk overriding a large VSD can be easily confused with TOF/PA, PA/VSD, aortic atresia with VSD, and DORV given in these situations the PA or aorta may be hypoplastic and difficult to visualize.
- In TA, the branch arteries arise from the single great artery, but in PA either the branch pulmonary arteries arise from the ductus arteriosus, which will have retrograde flow, or the branch pulmonary arteries are hypoplastic and the lungs are supplied via MAPCAs. In aortic atresia, there will be a severely hypoplastic ascending aorta and arch, and flow in the arch will be retrograde from the ductus.
- In TA, PDA is typically absent. Therefore, if both ductal and aortic arches present, other differential diagnoses must be considered.
- Making distinction between above mentioned cases and TA is clinically important because pulmonary or aortic atresia are ductal dependent lesions for systemic or pulmonary circulation, therefore would need PGE postnatally for ductal patency, whereas TA typically is not (unless there is arch obstruction/interruption).
- In approximately 1% of cases, truncus arteriosus is associated with severe coarctation of the aorta or interrupted aortic arch (IAA) (Van Praagh type A4). In these cases, the ductus arteriosus is present and is the primary source of blood flow to the lower body.
- TA with IAA cases are more common than the cases with coarctation of the aorta. In the setting of TA with IAA, ductus is usually the only arch present whereas in cases of coarctation, two arches may be present with the aortic arch being hypoplastic.
Fetal Surveillance:
- Assess truncal valve function for worsening regurgitation or evidence of stenosis
- Assess the growth of branch pulmonary arteries
- Assess flow across the ventricular septal defect
- If there is a concern for severe coarctation of the aorta or IAA (Van Praagh Type A4, it is important to monitor flow in the ductus arteriosus
Prostaglandins (PGE):
- Usually not needed in the most common forms of TA
- PGE is only needed if there suspected coarctation of the aorta or IAA
References:
-
McElhinney, D. B., MD. (2015, January 14). Truncus Arteriosus. Retrieved from http://emedicine.medscape.com/article/892489-overview#a4
-
Sojak, V., & Lugo, J. (2012, June 14). Surgery for truncus arteriosus. Retrieved from http://mmcts.oxfordjournals.org/content/2012/mms011.full
-
Lai, W. W. (2009). Truncus arteriosus. In Echocardiography in pediatric and congenital heart disease: From fetus to adult (pp. 385-392). Chichester, UK: Wiley-Blackwell.
-
Swanson TM, Selamet Tierney ES, Tworetzky W, et al: Truncus arteriosus: diagnostic accuracy, outcomes, and impact of prenatal diagnosis. Pediatr Cardiol 2009; 30: pp. 256-261
-
Volpe P, Paladini D, Marasini M, et al: Common arterial trunk in the fetus: characteristics, associations, and outcome in a multicentre series of 23 cases. Heart 2003; 89: pp. 1437-1441
-
Callen's Ultrasonography in Obstetrics and Gynecology, Sixth Edition
-
Egbe A, Uppu S, Lee S, et al: Changing prevalence of severe congenital heart disease: a population-based study. Pediatr Cardiol 2014; 35: pp. 1232-1238
-
Long J, Ramadhani T, and Mitchell LE: Epidemiology of nonsyndromic conotruncal heart defects in Texas, 1999-2004. Birth Defects Res A Clin Mol Teratol 2010; 88: pp. 971-979
-
Canfield MA, Honein MA, Yuskiv N, et al: National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Res A Clin Mol Teratol 2006; 76: pp. 747-756
-
Collett RW, and Edwards JE: Persistent truncus arteriosus; a classification according to anatomic types. Surg Clin North Am 1949; 29: pp. 1245-1270
-
Francalanci P, Gallo P, Dallapiccola B, et al: A genetic assessment of trisomy 21 in a patient with persistent truncus arteriosus who died 38 years ago. Am J Cardiol 1997; 79: pp. 245-247
-
Goldmuntz E, Clark BJ, Mitchell LE, et al: Frequency of 22q11 deletions in patients with conotruncal defects. J Am Coll Cardiol 1998; 32: pp. 492-498
-
Moore JW, Wight NE, Jones MC, and Krous HF: Truncus arteriosus associated with trisomy 18. Pediatr Cardiol 1994; 15: pp. 154-156