Ebstein Anomaly
J. Kevin Wilkes, MD
Overview and Natural History
Ebstein anomaly is a rare form of congenital heart disease in which the tricuspid valve annulus is apically displaced producing to variable degrees heart failure. The septal and posterior leaflets of the tricuspid valve fail to delaminate from the myocardium in development, leading to annular displacement and atrialization of the right ventricle (RV) that produces RV dilation and dysfunction. The anterior tricuspid valve leaflet is often redundant and commonly described as “sail-like.” This unbalanced deformation leads to a rotational displacement of the tricuspid valve into the right ventricular outflow tract (RVOT) causing a variable degrees of RVOT obstruction. The severity of disease can vary and is dependent on the degree of valve displacement, degree of atrialized RV, RVOT obstruction, and ventricular dysfunction. Initial presentation can vary from prenatal to late adulthood.
Epidemiology
Prevalence has been described from 0.5-24 per 100,000 live births and is equally distributed among genders. Associated cardiac lesions include atrial septal defect, pulmonary stenosis or atresia, and left sided lesions such as subaortic stenosis, bicuspid aortic valve, or left ventricular dysfunction depending on the severity of RV compression. Accessory pathways leading to ventricular pre-excitation are present in up to 30% of patients.
Genetics
Inheritance is likely multifactorial; affected by genetic, environmental, and reproductive exposures. While maternal lithium exposure early in pregnancy was initially thought to be associated with a marked increase in risk of Ebstein, newer studies have demonstrated a lesser impact.
Classification
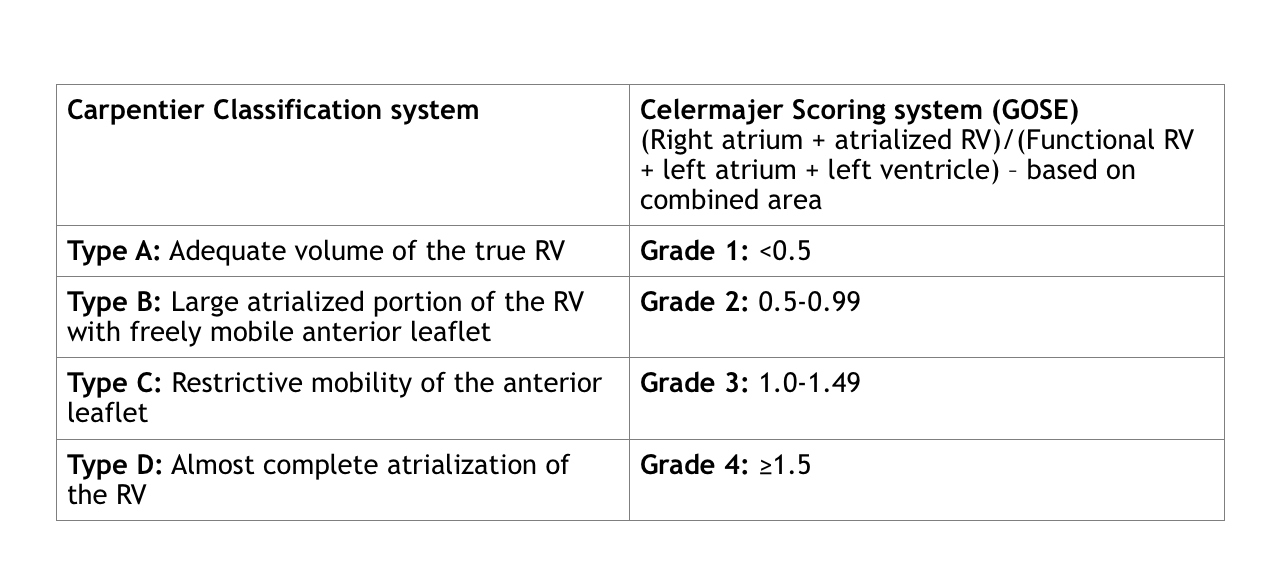
Classification systems include the qualitative Carpentier and quantitative Celermajer (Great Ormond Street Echocardiography (GOSE)) systems:
Hemodynamics
Physiology depends on the severity of the RVOT obstruction/pulmonary stenosis, degree of atrialized RV and RV dysfunction. Severe pulmonary stenosis or pulmonary atresia presents at birth with cyanosis with retrograde filling of the branch pulmonary arteries from the patent ductus arteriosus. Despite the absence of prograde flow across the pulmonary valve, there can be pulmonary regurgitation in “functional” pulmonary atresia.
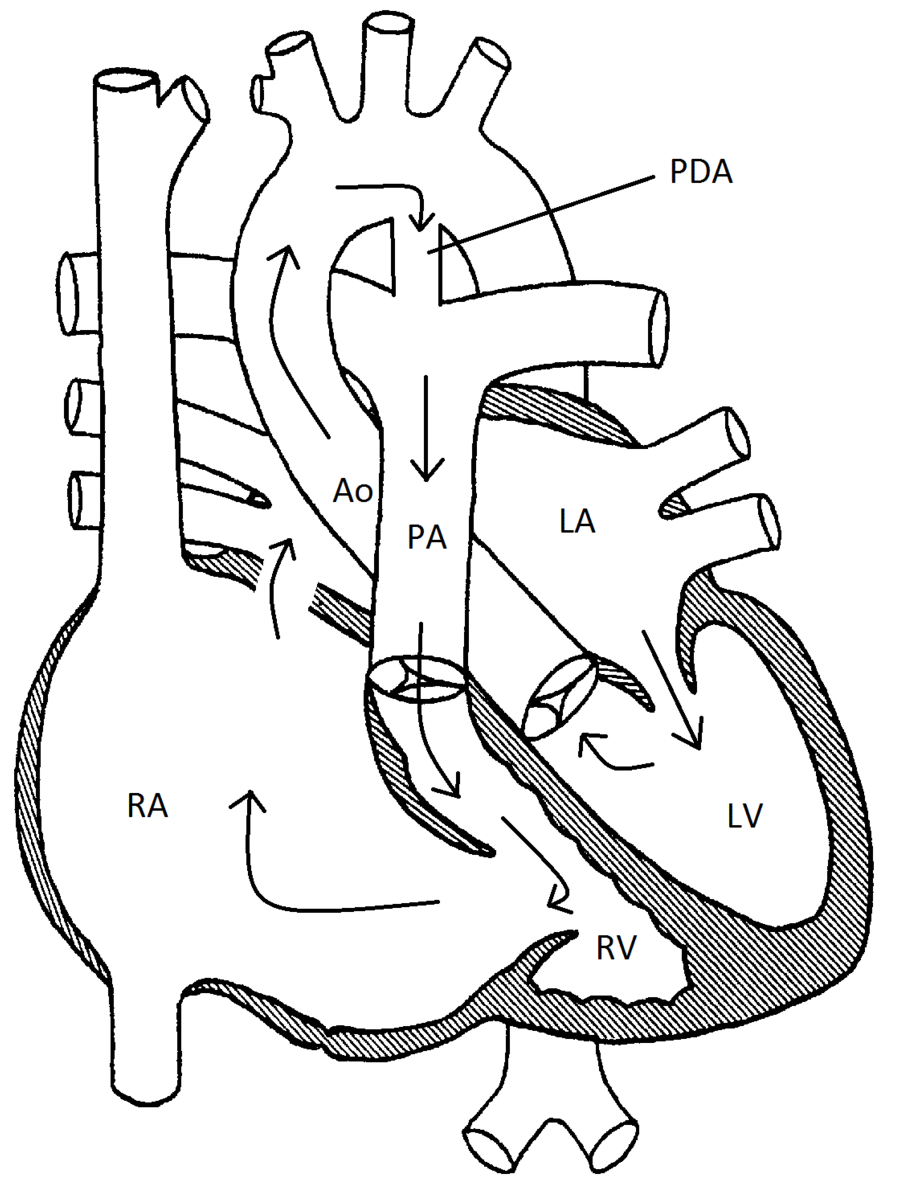
There is a high risk cohort of patients who demonstrate circular shunt physiology in the presence of severe tricuspid regurgitation, right-to-left flow across the atrial septal defect, prograde flow through the left ventricular outflow tract with subsequent retrograde flow from the patent ductus arteriosus into the MPA with severe pulmonary regurgitation with flow back into the right ventricle. This circular shunt may lead to rapid deterioration in utero or neonatally and carries a very guarded outcome.
Ebstein anomaly can vary in severity. The degree of RV atrialization dictates the amount of RV dilation and dysfunction. A severely atrialized right ventricle unable to generate the appropriate degree of antegrade flow in utero may lead to pulmonary valve hypoplasia/atresia. RV dysfunction varies from inability to generate prograde flow across the pulmonary valve during the neonatal period to a reasonably well developed pulmonary valve and branch pulmonary arteries (mild variants).
Anatomic diagram representing a circular shunt in Ebstein Anomaly
Goals of Echocardiography Exam
- Apical displacement of the septal leaflet of the tricuspid valve is measured as an index of the mitral valve annulus septal attachment (measurement obtained in the apical 4-chamber view in either systole or diastole and divided by patient’s BSA). An index of ≥8mm/m2 is supportive of the diagnosis of Ebstein anomaly.
- Morphology of tricuspid valve (size (Z-score), dysplastic thickened and rolled leaflets, fenestrations, multiple orifices, shortened chordae, underdeveloped papillary muscles, restricted mobility/tethering, or stenosis).
- Assessment of tricuspid regurgitation by color and Spectral Doppler (can be difficult to quantify given compliance of the atrialized RV and multiple jets of regurgitation. Hepatic vein flow reversal and width of the vena contract cannot reliably be used).
- Assessment of tricuspid stenosis with measurement of maximal velocity across the tricuspid valve (normal <0.8 meters/sec). Calculate maximal and mean diastolic transvalvular pressure gradient.
- Assess for pulmonary stenosis (should pulmonary stenosis be present, assess flow and degree of pulmonary valve hypoplasia as well as size of MPA and branch pulmonary arteries)
- Assess for presence of pulmonary atresia. The presence of pulmonary valve regurgitation (PR is indicative of functional pulmonary atresia), along with severity of RVOT obstruction, is an important characteristic when distinguishing “functional” from true pulmonary atresia. The direction of flow across a patent ductus arteriosus can also help determine adequacy of pulmonary valve prograde flow.
- Assessment for a PFO/ASD as the presence and direction of flow across an atrial septal defect or PFO can portend risk of circular shunt or paradoxical emboli.
- Assess for circular shunt physiology (requires presence of severe TR, PFO/ASD, PDA and pulmonary regurgitation).
- The RV size and function (Tei index, fractional area change) and the effect it has on the interventricular septum is important when evaluating the left ventricular size and function. Geometric distortion of the right and left ventricles makes quantification of function challenging.
- Assess anatomic severity through calculation of chamber area ratio:
- Chamber Area Ratio (measured at end diastole in apical 4 chamber view): (RA + aRV)/(RV + LA + LV)
- RA = Area of the right atrium
- aRV = Area of atrialized portion of RV
- RV = Area of the right ventricle
- LV = Area of the left ventricle
- LA = Area of the left atrium
- A ratio of ≥1 in neonate indicates a very poor prognosis