Tetralogy of Fallot
Hasti Sanandajifar, MD
Overview and Natural History
Tetralogy of Fallot (TOF) encompasses a spectrum of cardiac defects that stem from anterocephalad deviation of the outlet septum, along with abnormal septoparietal trabeculations which contribute to right ventricular infundibular narrowing. This leads to the four well-known components of TOF:
- Anterior malalignment ventricular septal defect (VSD)
- Aortic override over the muscular septum
- Variable degrees of subvalvar, valvar, and supravalvar pulmonary stenosis
- Right ventricular (RV) infundibular narrowing and RV hypertrophy.
Patients with TOF can be pink, have baseline cyanosis, or experience hypercyanotic spells (see hemodynamics below). Their physiology depends on the degree of right ventricular outflow tract (RVOT) obstruction and pulmonary stenosis. Risk of hypercyanotic spells increases over time, as RV infundibular muscles further hypertrophy.
In the current era, TOF is repaired during infancy with an excellent prognosis. Life expectancy for unrepaired TOF is low, historically cited as < 50% by 3 years of age and < 25% by 10 years.
Epidemiology
TOF is the most common form of cyanotic congenital heart disease. It comprises approximately 3.5% of infants born with congenital heart disease and 8-10% of cyanotic cases. Worldwide reported prevalence of TOF is 0.28 per 1,000 live births. There is no gender predilection.
Genetics
Approximately 25% of patients with TOF have a chromosomal abnormality, most commonly 22q11 deletion (DiGeorge) syndrome followed by trisomy 21. As with other conotruncal defects, TOF is associated with abnormal neural crest cell migration; 22q11 deletion is seen in about 20% of patients with TOF with pulmonary stenosis and in more than 40% of patients with TOF with pulmonary atresia.
Most cases are sporadic. However, children who have parents or siblings with TOF have a significantly higher risk of congenital heart disease.
Types/Anatomy
TOF presents as a spectrum of severity, from TOF with mild pulmonary stenosis to TOF with pulmonary atresia. Another rare variant is TOF with absent pulmonary valve, which is a distinct disease entity and discussed separately.
The VSD:
One of the important aspects of TOF anatomy is the location of the VSD. The VSD in TOF is always located in the outlet, within the limbs of the septomarginal trabeculation, and the outlet septum is deviated anteriorly. The superior boundary of the VSD is most commonly the outlet septum which is exclusively an RV structure.
Types of VSDs in TOF:
- Perimembranous Outlet VSD (60-70%): located between the limbs of the septal band and extend to the area of fibrous continuity between the tricuspid and aortic valves. This is behind the septal leaflet of the tricuspid valve and below the commissure between the right and noncoronary leaflets of the aortic valve. The posteroinferior rim is fibrous.
- Muscular Outlet VSD (20-30%): have a muscular posteroinferior limb. They are not in fibrous continuity with the AV septum and are located away from the AV conduction pathway and above the posteroinferior limb of the septal band
- Doubly Committed Subarterial VSD (rare): characterized by complete absence of the outlet (or conal) septum and results in aortopulmonary fibrous continuity. Occurs with hypoplasia of the subpulmonary infundibulum and hypertrophy of the septoparietal band when associated with TOF. Posteroinferior rim is typically muscular, but it can have perimembranous extension.
- Other:
- Restrictive VSD (rare, ~ 1.5% of TOF cases): typical mechanism impingement of tricuspid valve tissue into VSD causing restriction; usually associated with suprasystemic RV pressures
- TOF with atrioventricular septal defect (1.7% of TOF cases): leads to confluent inlet and outlet VSD. Look for genetic and extracardiac abnormalities.
RVOT obstruction:
- Subvalvar PS: Prominent septoparietal muscle bands or trabeculations that tend to undergo progressive hypertrophy postnatally
- Pulmonary valve (PV) dysplasia:
- Thickened immobile leaflets
- Commissural fusion, bicuspid in 75% of cases
- Possible tethering of leaflets to the pulmonary artery wall
- Hypoplastic pulmonary valve annulus
- Supravalvar PS and branch pulmonary artery (PA) stenoses
- Hypoplasia or discrete stenoses within the main PA (MPA) trunk and/or branch PAs
- Possible ductal shelf in left PA (LPA)
Aortic Root, Arch, and Coronaries:
- Clockwise rotation of developing outflow tract and aortic root
- Aortic root positioned slightly more rightward and anteriorly than usual
- Coronary origins clockwise rotated along with the aortic root
- Coronary artery branching
- Larger conal branch from the right coronary artery (RCA) crossing the RVOT in 6-7%.
- Branching anomalies
- Left anterior descending (LAD) from RCA or duplicated LAD (5-7%)
- Aortic root is typically dilated
- Arch
- Right aortic arch (RAA) with mirror-image branching in 25% of TOF
- Aberrant subclavian artery (10%)
Venous abnormalities:
- Presence of persistent left superior vena cava (up to 10%)
Hemodynamics
Patients with TOF can be pink or cyanotic at baseline, based on the degree of right ventricular outflow tract (RVOT) obstruction and pulmonary stenosis. They may also have hypercyanotic spells.
“Pink” TOF has adequate pulmonary blood flow and can be monitored clinically and by serial echocardiography until full repair. In some cases, if pulmonary stenosis is very mild, this physiologically may act more like a VSD (with associated symptoms of pulmonary overcirculation).
“Cyanotic” TOF has ductal dependent pulmonary blood flow and requires PGE administration following birth to keep the PDA open to ensure sufficient pulmonary blood flow until intervention or repair is performed.
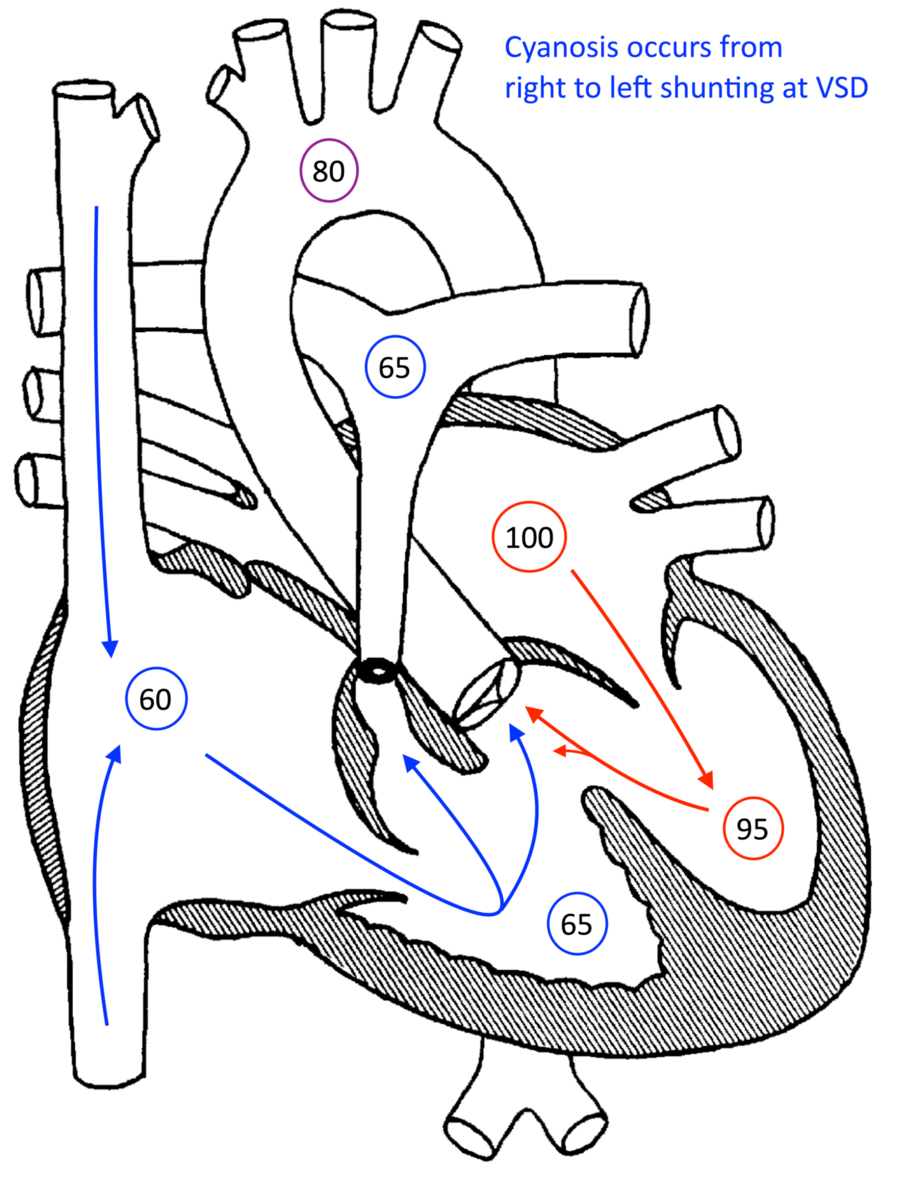
Hypercyanotic spells are caused by dynamic RVOT obstruction secondary to RV infundibular narrowing as the ventricle contracts. This causes right-to-left shunting at the VSD and decreased pulmonary blood flow. Hypercyanotic spells can be associated with tachycardia, catecholamine release, decreased right ventricular preload, and acute reduction in systemic vascular resistance; as such, they can occur in the setting of fever, stress, crying, agitation, or dehydration. They can occur at any age based on the degree of infundibular narrowing, but they become more common after 6-12 months of age as the infundibular muscles further hypertrophy.
Repair
Cyanotic or spelling patients may need a palliative intervention in the newborn period. Options include: percutaneous stenting of the patent ductus arteriosus or RVOT or, alternatively, a surgical systemic to pulmonary shunt. In select cases, some centers may perform an earlier neonatal complete repair.
Complete repair is most commonly performed at 4-6 months of age and consists of VSD closure (committing the aorta to the left ventricle) and relief of the RVOT obstruction. This is accomplished via one of three main paths: 1) a “valve-sparing” repair which is comprised of RVOT muscle bundle resection and possible patch plasty of the pulmonary arteries if the pulmonary valve (PV) size and function are adequate, 2) a transannular patch from the RV to the MPA (leaving “free” pulmonary regurgitation), or 3) a valved RV to PA conduit in cases of pulmonary atresia or if the PV annulus is too small for patching anteriorly. Post-operative mortality is < 2% based on STS data from 2019.
Patients with repaired TOF have excellent survival, exceeding 90% at ages 40s-50s. Long-term consequences after repair include severe pulmonary regurgitation necessitating pulmonary valve replacement (percutaneous or surgical) as well as atrial and ventricular arrhythmias requiring treatment.
Goals of Echocardiography Exam
Echocardiography can define the VSD size and location, identify the degree of fixed and dynamic RVOT obstruction, and evaluate the general cardiac anatomy and function. Fixed obstruction can be due to subvalvar, valvar, and/or supravalvar stenosis. Dynamic obstruction occurs as the hypertrophied RV infundibular muscle contracts, causing further RVOT obstruction over the course of systole.
This information can help assess the patient’s physiology and, in some cases, identify patients at higher risk for hypercyanotic spells. Assessing an accurate pulmonary outflow gradient can be challenging in neonatal life, as this depends on the pulmonary vascular resistance (PVR), which decreases over the first few weeks and months of life. The measured gradient also depends on the presence and size of a patent ductus arteriosus; therefore it is helpful to monitor RVOT gradient and patient’s systemic oxygen saturations as the PVR decreases and the PDA constricts or closes. Thus, serial echocardiograms can give us many clues regarding the TOF physiology, but ultimately the categorization of “pink” vs “blue” tetralogy is a clinical diagnosis.
Echo is also used to guide surgical repair, with particular attention to VSD size and location, degree of RVOT obstruction, pulmonary valve and pulmonary artery size and stenosis, presence of coronary artery crossing the RVOT, and diagnosis of other associated lesions that may need to be addressed concurrently. In terms of VSD closure, differentiating between perimembranous outlet vs. muscular outlet vs. doubly committed VSD can affect how the surgeon performs the repair and the suture lines chosen in order to avoid damage to the cardiac conduction system.
Complete cardiac anatomic evaluation with specific attention to the following:
- VSD morphology:
- perimembranous outlet, muscular outlet, or doubly committed defect with anterior malalignment of outlet septum (fibrous remnant in doubly committed)
- presence of atrioventricular septal defect
- any additional muscular VSDs
- RVOT obstruction: location and degree
- Presence of dynamic subvalvar obstruction
- Pulmonary valve size and morphology
- Main and branch pulmonary artery size and morphology
- Subaortic and aortic valve morphology and function
- Sources of pulmonary blood flow (PDA, collaterals, etc.)
- Arch sidedness and branching
- Coronary artery anatomy
- Presence of persistent left superior vena cava with/without bridging vein
- Presence/absence of the thymus (associated with 22q11.2 deletion syndrome)
Detailed Evaluation of Pulmonary Outflow Obstruction
- Dynamic obstruction in the RVOT has a late-peaking “lobster claw” or dagger-shaped Doppler signal
- Fixed valvar or supravalvar obstruction has mid-systolic peak, more symmetric Doppler signal
- Obstruction in series (i.e. multiple levels of obstruction) can be difficult to differentiate by Doppler echocardiography but may have overlapping Doppler patterns (superimposed fixed and dynamic signals)
- Degree of obstruction* defined as:
|
Degree of obstruction |
Doppler velocity |
Gradient across RVOT/PV |
|
Mild |
< 3 m/s |
< 36 mmHg |
|
Moderate |
3 - 4 m/s |
36 – 64 mmHg |
|
Severe |
> 4 m/s |
> 64 mmHg |
*Note dependence on pulmonary vascular resistance, presence of PDA, systemic blood pressure in presence of large VSD in unrepaired TOF
References:
- Lai WW, Mertens L, Cohen M, Geva T, editors. Echocardiography in pediatric and congenital heart disease: from fetus to adult. Second edition. Chichester, West Sussex ; Hoboken, NJ: John Wiley & Sons Inc; 2015.
- Allen HD, Shaddy RE, Penny DJ, Cetta F, Feltes TF. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult. 2015.
- Park MK. Park’s pediatric cardiology for practitioners: get full access and more at expertconsult.com. 6. ed. Philadelphia, PA: Elsevier Saunders; 2014.
- Apitz C, Anderson RH, Redington AN. Tetralogy of Fallot with Pulmonary Stenosis. In: Paediatric Cardiology. Elsevier; 2010. p. 753–73.
- Kliegman R, Behrman RE, Nelson WE, editors. Nelson textbook of pediatrics. Edition 20. Phialdelphia, PA: Elsevier; 2016. 2 p.
- Handler SS, Ginde S, Bergstrom CP, Woods RK. Tetralogy of Fallot With and Without Pulmonary Atresia. In: Critical Heart Disease in Infants and Children. Elsevier; 2019. p. 705-719.e4.
- Lopez L, Houyel L, Colan SD, Anderson RH, Béland MJ, Aiello VD, et al. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases—Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. The Annals of Thoracic Surgery. 2018 Nov;106(5):1578–89.
- Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM, et al. Multimodality Imaging Guidelines for Patients with Repaired Tetralogy of Fallot: A Report from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2014 Feb;27(2):111–41.
- Mostefa-Kara M, Bonnet D, Belli E, Fadel E, Houyel L. Anatomy of the ventricular septal defect in outflow tract defects: Similarities and differences. The Journal of Thoracic and Cardiovascular Surgery. 2015 Mar;149(3):682-688.e1.
- Ebadi A, Spicer DE, Backer CL, Fricker FJ, Anderson RH. Double-outlet right ventricle revisited. The Journal of Thoracic and Cardiovascular Surgery. 2017 Aug;154(2):598–604.
- McKenzie ED, Maskatia SA, Mery C. Surgical Management of Tetralogy of Fallot: In Defense of the Infundibulum. Seminars in Thoracic and Cardiovascular Surgery. 2013 Sep 1;25(3):206–12.
- Flanagan MF, Foran RB, Van Praagh R, Jonas R, Sanders SP. Tetralogy of fallot with obstruction of the ventricular septal defect: Spectrum of echocardiographic findings. Journal of the American College of Cardiology. 1988 Feb;11(2):386–95.
- Kulkarni S, Jain S, Kasar P, Garekar S, Joshi S. Retroaortic left innominate vein - Incidence, association with congenital heart defects, embryology, and clinical significance. Ann Pediatr Card. 2008;1(2):139.
- Bertranou EG, Blackstone EH, Hazelrig JB, Turner ME, Kirklin JW. Life expectancy without surgery in tetralogy of fallot. The American Journal of Cardiology. 1978 Sep 1;42(3):458–66.
- Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades☆. European Journal of Cardio-Thoracic Surgery. 2009 Jan;35(1):156–64.
- Dennis M, Moore B, Kotchetkova I, Pressley L, Cordina R, Celermajer DS. Adults with repaired tetralogy: low mortality but high morbidity up to middle age. Open Heart. 2017 Mar;4(1):e000564.
- https://www.sts.org/sites/default/files/Congenital-STSExecSummary_AllPatients.pdf