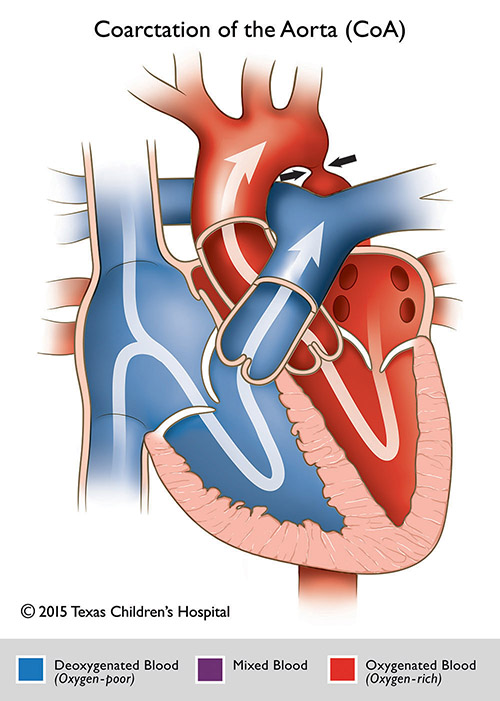
Coarctation of the Aorta
Tyler Fick, MD
Overview and Natural History:
Coarctation of the aorta is a narrowing of the aorta most commonly in the region distal to the head and neck vessels in a region called the aortic isthmus. This site of coarctation, also termed “juxtaductal” aorta is the portion of the aorta in close proximity to where the ductus arteriosus inserts. The ductus arteriosus is a blood vessel present in the fetus which typically closes postnatally in the first few hours to days of life. Once the PDA closes, this can cause aortic narrowing. In addition, to having a discrete coarctation, some patients may demonstrate a long segment coarctation or diffuse hypoplasia of the transverse arch.
Epidemiology
Coarctation is found in 6% to 8% of patients with congenital heart disease. Associated anomalies include bicuspid aortic valve, ventricular septal defect, small left sided structures (also known as Shone’s variant/complex) as well as variants of single ventricle anatomy. Coarctation of the aorta also accounts for a small percentage of children diagnosed later in life who present with systemic hypertension.
Embryology and Genetics:
The aortic arch and head and neck vessels develop between 6 to 8 weeks of gestation. The embryologic 3rd aortic arch persists as the common carotid arteries. The left 4th arch forms the thoracic aortic arch and aortic isthmus. The right 4th aortic arch normally involutes. The left 6th aortic arch develops into the PDA. Coarctation is a result of abnormal embryologic development of the left 4th and 6th aortic arches. Two prominent theories exist to explain coarctation:
- Ductal Tissue Theory: Coarctation is a result of migration of ductal tissue into the region of the aortic isthmus with subsequent constriction and narrowing of the aortic lumen.
- Hemodynamic Theory: Coarctation develops secondary to hemodynamic abnormalities in which there is decreased blood flow during fetal life through the aortic arch. Certain CHD lesions (such as VSD, aortic stenosis and arch hypoplasia) diminish flow through the aortic isthmus and contribute to development of coarctation.
There is an increased prevalence of coarctation in various genetic syndromes such as Turners syndrome (XO), Williams Syndrome, Noonan Syndrome, Rubenstein-Taybi syndrome, Alagille Syndrome, Neurofibromatosis, Kabuki Syndrome and PHACES syndrome. There is a slight male predominance. Offspring and other first-degree relatives diagnosed with an obstructive left-sided cardiac lesion are at ten times the risk of coarctation and other cardiac lesions
Hemodynamics:
In fetal life, hemodynamics are rarely disturbed given the small percentage of combined ventricular output that travels through the aortic isthmus. Following birth and closure of the PFO and PDA, all cardiac output must now cross the aortic isthmus. Depending on the severity of coarctation, presentation can be variable from mild hypertension (mild coarctation) to LV systolic dysfunction with congestive heart failure and cardiogenic shock (more severe coarctation).
In the setting of severe postnatal coarctation following ductal closure, newborns have decreased stroke volume, increased left ventricular end diastolic pressure and elevated left atrial pressure which can progress to pulmonary venous congestion and pulmonary hypertension. In the setting of decreased cardiac output there is diminished myocardial and systemic perfusion with the development of lactic acidosis and end organ injury (kidney, liver, bowel).
Goals of Echocardiographic Exam:
- Coarctation best visualized from suprasternal notch view with 2D, color and spectral Doppler imaging. Obtain a peak velocity with a mean pressure gradient across transverse arch and isthmus (site of coarctation)
- Flow will often be high velocity with persistence of flow into diastole (diastolic runoff)
- Identify arch sidedness and branching pattern of the head and neck vessels and assess for distal displacement of the left subclavian artery (often seen) from the suprasternal notch view
- Obtain precise 2D aortic arch measurements along with z-scores (ascending aorta, proximal transverse arch, distal transverse arch, aortic isthmus). Determine if there is an isolated coarctation or diffuse transverse arch hypoplasia (may determine if surgeon approaches surgical repair from a median sternotomy versus lateral thoracotomy)
- Arch Dimensions
- Proximal transverse arch: Diameter of aortic arch between innominate artery and left carotid artery
- Distal transverse arch: Diameter of aortic arch between left carotid artery and left subclavian artery
- Aortic isthmus: Diameter of aortic arch distal to the left subclavian artery
- Normal newborn arch measurements as a rough estimation: weight in kg + 1 for proximal arch, weight in kg for distal arch, then > 2-3 mm at the isthmus
- In setting of coarctation may observe dampened/blunted flow patterns (reduced velocity) with flow persistence into diastole (diastolic runoff) in abdominal aortic doppler from subcostal assessment
- It is important to perform pulse-wave doppler assessment via an angle most parallel and least perpendicular to the angle of the abdominal aorta.
- Detailed assessment of left-sided structures (aortic valve, mitral valve, left ventricle) for hypoplasia (with z-scores) and assess for abnormalities such as bicuspid/hypoplastic aortic valve, abnormal or hypoplastic mitral valve, Left ventricular hypoplasia.
- Assess the ventricular septum for ventricular septal defects with detailed sweeps (2D and color and spectral Doppler should VSD be present) from parasternal, apical and subcostal views.
- Evaluate for the presence, size and determine flow pattern in ductus arteriosus by 2D, color and spectral Doppler from suprasternal notch (three vessel view) and parasternal views. In coarctation, PDA should be right to left or bidirectional.
- Cannot rule out a coarctation of the aorta in the presence of a PDA. Often the PDA must constrict before a coarctation becomes evident.
- Assess for right/left heart disproportion (RVH/right ventricular dilation) as well as varying degrees of left ventricular hypoplasia
- Evaluation of atrial communication if present along with size, direction and mean pressure gradient
- Evaluate for pulmonary hypertension (right heart size, tricuspid and pulmonary regurgitation Doppler patterns and septal configuration from parasternal short axis views)
- Detailed assessment of right (TAPSE, RV fractional area change) and left ventricular systolic function (m-mode, bullet and Simpson’s biplane) as critical coarctation can result in depressed ventricular function.
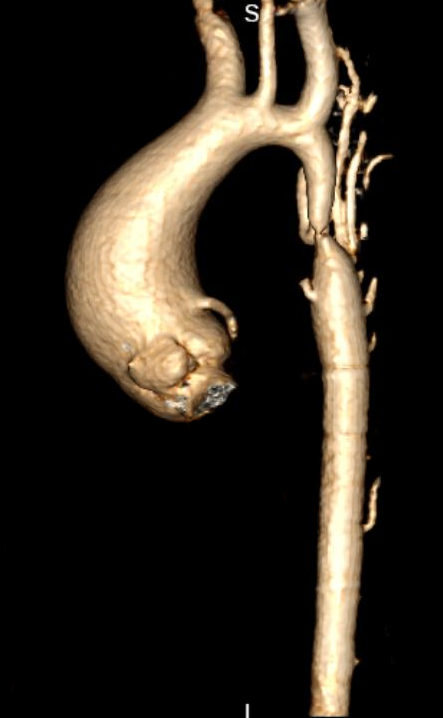
CT scan of a patient with a bicuspid aortic valve with a severly dilated ascending aorta with severe coarctation of the aorta
References:
- Moss, Arthur J., and Hugh D. Allen. Moss and Adams’ Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult. 9th edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2016.
- Anderson, R., Baker E., Penny, D., Redington, A., Rigby M. & Wernovsky, G. Paediatric Cardiology. Philadelphia: Churchill Livingstone, 2010.
- Jaquiss, R. Coarctation of the Aorta: End-to-End Anastomosis. Operative Techniques in Thoracic and Cardiovascular Surgery, Vol 7, No 1, 2002; 2-10
- Mery, C et al. Contemporary Results of Aortic Coarctation Repair Through Left Thoracotomy. Ann Thorac Surg, 2015; 1039-46.
- Fesseha, A et al. Neonates with Aortic Coarctation and Cardiogenic Shock: Presentation and Outcomes. Ann Thorac Surg, 2005; 1650-5.
- Yetman AT, Starr L, Sanmann J, Wilde M, Murray M, Cramer JW. Clinical and Echocardiographic Prevalence and Detection of Congenital and Acquired Cardiac Abnormalities in Girls and Women with the Turner Syndrome. Am. J. Cardiol. 2018 Jul 15;122(2):327-330.
- Images above used with permission from Texas Children's Hospital and courtesy of the division of cardiothoracic surgery