Transposition of the Great Arteries (D-TGA)
Raysa Morales-Demori, MD
Overview
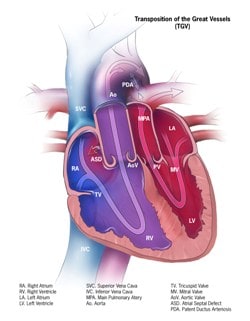
Transposition of the great arteries (D-TGA) is a conotruncal abnormality characterized by discordant ventricular-arterial connections. In D-TGA, the aorta arises from the right ventricle and pulmonary artery arises from the left ventricle. The aorta is anterior and rightward. The pulmonary and aortic circulations are in parallel instead of in series.
Epidemiology
D-TGA occurs in ~31.5 in 100,000 live births. It is the 10th most common form of congenital heart disease, and the second most common cyanotic heart disease following Tetralogy of Fallot. There is a male predominance (2:1 male to female ratio).
Genetics
It is usually not hereditary, however, infants of diabetic mothers are at increased risk.
Associated lesions
- Ventricular septum: Intact (60%) vs Ventricular septal defect (VSD) (40%).
- Pulmonary stenosis.
- TGA/VSD can be associated with overriding or straddling AV valves
- Hypoplastic aortic arch/Coarctation of aorta.
- Right ventricular outflow tract obstruction.
- Left ventricular outflow tract obstruction.
- Coronary artery abnormalities (RCA= right coronary artery, LCA = left coronary artery, LAD= left anterior descending)
- Usual (RCA off right posterior sinus and LCA off left posterior sinus of Valsalva)
- Circumflex from the RCA
- Single LCA or RCA
- Inverted CAs (both RCA and LCA inverted or inverted RCA and circumflex)
- Intramural coronary arteries (can be LCA, LAD or RCA)
Diagnosis
- Prenatally: Fetal echocardiogram. Apical 4 chamber sweep demonstrates parallel great arteries with a lack of crossing of the great vessels. This diagnosis can be missed if the fetal ultrasound does not sweep to the outflow tracts, highlighting the importance of this view.
Clinical Presentation
- Cyanosis: Usually diagnosis happens shortly after birth due to parallel systemic and pulmonary circulatory systems. Neonates will be more cyanotic if there is poor intracardiac mixing between the parallel circulations (restrictive or intact atrial septum). If cyanosis is severe, neonates will need an urgent balloon atrial septostomy to improve mixing +/- prostaglandins to keep PDA patent (another site of mixing).
- Reverse Differential Cyanosis: In some cases, neonates can present with reverse differential cyanosis (defined as higher saturation in lower extremities compared to upper extremities) from right to left shunting at the PDA in cases of D-TGA with coarctation, D-TGA with pulmonary hypertension or D-TGA with interrupted aortic arch (IAA).
Goals of the Echocardiography Exam
- Establish origin and position of the great arteries from multiple views with good full sweeps (parasternal long axis, short axis, apical 4 chamber, subcostal)
- Interrogate the atrial septum to assess for the size/presence of a PFO/ASD (2D, color, spectral Doppler). Determine whether ASD is restrictive as this would be an indication for urgent balloon atrial septostomy
- Assess for presence of PDA or VSD (PGEs may be needed to keep PDA open if mixing not adequate at atrial septum)
- Assess right or left ventricular outflow tracts for narrowing/stenosis
- Assess pulmonary and aortic valve size and morphology, assess for pulmonary or aortic stenosis or regurgitation (2D, color, spectral)
- Assess coronary artery anatomy (abnormal coronary artery branching patterns are common in D-TGA)
- Assess aortic arch for evidence of hypoplasia/coarctation
- Assess ventricular size and function
Surgery/Procedures
- Cardiac catheterization: Usually done during the first day of life in setting of postnatal cyanosis
- Balloon atrial septostomy: In case of insufficient blood mixing/shunting and restrictive PFO or intact atrial septum.
- Cardiac Surgery: Usually done during the first week of life, to avoid left ventricular deconditioning and risk of pulmonary hypertension
- D-TGA with intact ventricular septum or small VSD
- Arterial switch operation (ASO or Jatene operation) with LeCompte maneuvre: Currently is the most frequent procedure. First time successfully performed in 1975, and it is the preferred surgery (if possible) since 1980’s. It consists in transecting the great arteries and switching them to the other semilunar valve. This way, the aorta with coronary arteries get translocated to the native-pulmonary artery valve and the pulmonary artery gets translocated to the native-aortic valve. LeCompte maneuver consists in positioning of the pulmonary artey and main right and left branches anteriorly to the aorta to avoid distortion.
- Atrial switch operation, also known as Mustard or Senning procedure: They were the palliative procedure of choice between 1960-1980’s.
- Mustard procedure: Utilizes pericardial patch to baffle systemic venous flow to mitral valve and pulmonary venous flow to tricuspid valve
- Senning procedure: Same principle as Mustard procedure, but utilizes atrial tissue to baffle systemic venous return to mitral valve and pulmonary venous return to tricuspid.
- Complications of these procedures include: Right ventricular dysfunction, found in 10% of the patients 1 year post surgery; tricuspid regurgitation, sinus node dysfunction, intraatrial reentry tachycardia, and baffle obstruction
- D-TGA with VSD and pulmonary stenosis:
- Rastelli procedure: It consists of baffling the aorta to the left ventricle and using a right ventricle to pulmonary artery conduit. Baffling depends in the side of the VSD. Complications: Left ventricular outflow tract obstruction, conduit stenosis or regurgitation, formation of pseudoaneurysm next to the conduit.
- Nikaidoh procedure: It consists in mobilizing the aortic root and coronary arteries posteriorly, doing a LeCompte maneuver, resecting the main pulmonary artery and replacing it with a right ventricle to pulmonary artery conduit and closing the VSD with a patch.
- REV (Reparation a l’etage ventriculare) procedure: Main pulmonary artery and ascending aorta are detached, to perform a LeCompte maneuver. Ascending aorta is reattached to the aorta. Baffling of aorta to the left ventricle using the VSD. Oversewing of the pulmonary valve with detachment of the main pulmonary artery with direct anastomosis of the MPA to the RV (RV to PA connection with native tissue without a conduit).
- D-TGA with intact ventricular septum or small VSD